It is important to set expectations and educate patients about their treatment journey. Use these steps to get patients started

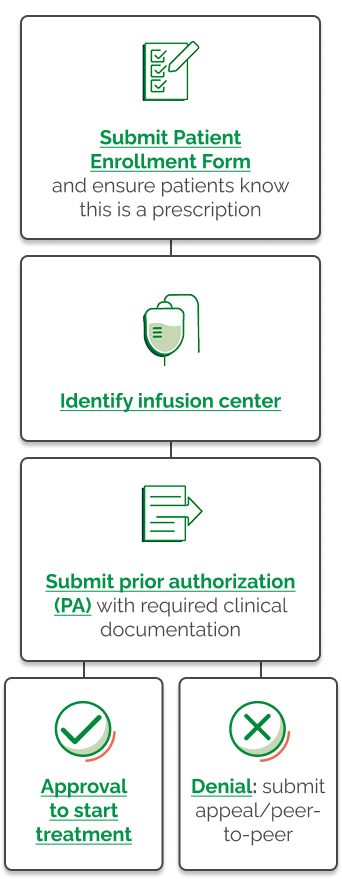 Submit Enrollment
Identify Infusion Center
Submit Prior Authorization
Approval to Start Treatment
Denial
Submit Enrollment
Identify Infusion Center
Submit Prior Authorization
Approval to Start Treatment
Denial
Read the Terms and Conditions of the Amgen Commercial Co-Pay Program.
*Individual patient access varies.
†Based on commercial plan analysis.
Terms and Conditions: Offer cannot be combined with any other rebate or coupon, free trial, or similar offer for the specified prescription. Not valid for prescriptions reimbursed in whole or in part by any government-funded program including but not limited to: Medicare, Medicare Part D, Medicaid, Medigap, VA, CHAMPUS, DOD, TRICARE, or any state, patient foundation, or other pharmaceutical program. Offer good only in the United States at participating specialty pharmacies or sites of care. Offer not valid where otherwise prohibited by law, for example by applicable state law prohibiting co-pay cards. Amgen reserves the right to rescind, revoke, or amend offer without notice. The selling, purchasing, trading, or counterfeiting of any co-pay card or benefits is prohibited by law. This co-pay program is not insurance and is not intended to substitute for insurance. Age for eligibility is dependent on product indication.
![]() Short chapters at your fingertips
Short chapters at your fingertips
Jump to what matters most ![]()
1. How TEPEZZA Works to Treat Thyroid Eye Disease
(0:28-01:51)
2. The Insurance Approval Process
(01:52-02:45)
3. Personalized Support with Amgen By Your Side
(02:46-03:35)
4. Getting your TEPEZZA
Infusion
(03:36-05:25)
5. The Possible Side Effects of TEPEZZA
(05:26-06:48)
6. How TEPEZZA Can Help Thyroid Eye Disease
(06:49-07:59)
Get to know your Thyroid Eye Disease treatment.
Thyroid Eye Disease or T-E-D, can be difficult to manage, but you haven't let it stop you.
You took control, got answers, and found the help you needed.
You and your doctor chose TEPEZZA. A prescription medicine used to treat T-E-D. TEPEZZA is the only medicine that treats T-E-D at a source.
Way to go! You're on your way.
Chapter 1, How TEPEZZA Works to Treat Thyroid Eye Disease.
To understand what causes T-E-D, you have to go behind the eye.
In the tissue behind the eye are cells that have receptors on their surface that act like switches. When you have T-E-D, your body's immune system attacks this tissue, which causes molecules known as autoantibodies to turn these switches on.
The muscles and the fat tissue swell pushing the eyeball outward, which your doctor may call proptosis.
Similarly, it can cause double vision, which your doctor may call diplopia. TEPEZZA is the first and only FDA-approved treatment for Thyroid Eye Disease.
Unlike other medicines, TEPEZZA treats a cause of T-E-D, not just the symptoms.
TEPEZZA is designed to bind to the switch and block it from turning on. Since TEPEZZA blocks the switches so they can't turn on, swelling of the muscle and fat tissue goes down.
This leads to reduced bulging and can also improve double vision and in some cases make it completely go away.
Chapter 2, The Insurance Approval Process.
Now that you and your doctor have decided on TEPEZZA, the next step is to gain approval from your insurance provider. TEPEZZA is a specialty medicine, which means it may require extra insurance approvals.
These approvals require steps like benefits investigation, and prior authorization that usually take between 30 and 90 days, but in some cases, may take longer.
If you're not familiar with these insurance terms, don't worry. In the next chapter of this video, you'll learn about support services available to help you through the TEPEZZA insurance approval process.
The developer of TEPEZZA believes costs should never stop someone from getting the medicine they need.
They're committed to helping you explore cost assistance options. That information will be in the next chapter as well.
Chapter 3, Personalized Support with Amgen By Your Side. Amgen By Your Side, a patient support program is here to help.
Once you and your doctor have decided TEPEZZA is right for you, you can sign up to be paired with the Patient Access Liaison or PAL for short.
PALs from the Amgen By Your Side team are available for personal, nonmedical, and logistical assistance throughout your treatment including discussing what your doctor may have explained about starting treatment, helping to understand the insurance process, explaining financial assistance options, helping you plan your first infusion, and more.
If you're interested, ask your doctor if you can sign the patient enrollment form before it's submitted to Amgen By Your Side. Once enrolled, you will receive a call from your PAL.
Chapter 4, Getting your TEPEZZA Infusion. TEPEZZA is an IV or intravenous medicine. This means it's delivered through a needle placed in your arm. The process of delivering an IV medicine into the body is called an infusion.
A full course of TEPEZZA treatment is 8 infusions. Each infusion is given one time every three weeks. That means the treatment with TEPEZZA will be ongoing for about five months.
TEPEZZA may be given at an infusion center, a type of clinic that specializes in giving infusions.
It may also be given at a doctor's office, hospital or at home. Check with both your insurance provider and your doctor to see if home infusion may be an option for you.
For your first infusion, having someone take you to and from the appointment is recommended.
When it's time to start your TEPEZZA treatment, you'll be taken to an infusion chair, which is a lot like a recliner. Once the IV is in, all you have to do is relax and receive the infusion.
Each infusion appointment usually lasts one and a half to two and a half hours, but will vary depending on where you get your infusion. During this time, books, music and movies can all provide a welcome distraction.
Karen W.: I was very scared. To avoid everything else that was on the road for me, the infusions didn't sound so bad for me. Then, of course, three weeks later, I went back for the second and it was the same thing. They would make me very comfortable. I didn't even think of it as oh my gosh, I'm sitting here getting an infusion. This is terrible. I thought, hey, you know, this isn't bad.
ANNCR: Chapter 5, The Possible Side Effects of TEPEZZA.
It's important to note that most of the side effects patients experienced in clinical trials were mild, uncomfortable, but didn't stop normal activity, or moderate, uncomfortable, and might have impacted normal activity.
Some patients experienced side effects that were severe, uncomfortable, and impacted normal activity.
Here's a list of the most common side effects of TEPEZZA and the percentage of people who experienced them in clinical studies.
Muscle cramps or spasms, 25% of patients; nausea, 17%; hair loss, 13%; diarrhea, 12%; tiredness, 12%; high blood sugar, 10%; hearing problems, 10%; changes in taste, 8%; headache, 8%; dry skin, 8%; weight loss, 6%; nail problems, 5%; changes in menstruation, 23%.
TEPEZZA may increase your blood sugar levels, so ask your doctor about developing a plan to have your blood sugar tested and tracked during treatment.
Always reach out to your doctor to discuss how to manage side effects.
Chapter 6. How TEPEZZA Can Help Thyroid Eye Disease.
In clinical studies, TEPEZZA has been proven to reduce eye bulging and improve double vision.
Some people had less eye bulging as soon as six weeks after starting treatment with TEPEZZA, and improvement continued over the full treatment course of 8 infusions.
Once you start seeing results with TEPEZZA, you may want to stop receiving infusions, but to get the full benefit, it is recommended you receive all 8 of them.
Jeanne T.: It was a slow process, but the bulging became less. The double vision was gone. To not have the bulgy eyes, to not look weird, it's really nice.
ANNCR: Follow TEPEZZA on Facebook or Instagram to join the community, get important updates, and stay connected.
Congratulations on taking the first step with TEPEZZA. Please listen to the important safety information.
USE
TEPEZZA is a prescription medicine used to treat Thyroid Eye Disease (TED), no matter if you’ve had TED for months or years.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about TEPEZZA?
Infusion reactions can happen during or within 24 hours after your infusion of TEPEZZA. If you have a reaction while receiving TEPEZZA, your doctor or nurse will slow or stop your infusion and treat your reaction. If you have a severe infusion reaction, your doctor may stop your treatment completely.
Tell your doctor or nurse right away if you have any of these symptoms during or after your treatment with TEPEZZA:
If you have inflammatory bowel disease (IBD), such as Crohn's disease or ulcerative colitis, TEPEZZA may make your IBD symptoms worse. Symptoms of worsening IBD may include: an increased number of loose stools with stomach pain or cramps, and blood in your stools. After each TEPEZZA infusion, tell your doctor right away if you have worsening IBD symptoms.
TEPEZZA may cause an increase in your blood sugar. Before starting treatment with TEPEZZA, tell your doctor if you are currently being treated for diabetes, know your blood sugar is high, or have been diagnosed with diabetes. It is important for you to take your treatments and follow an appropriate diet for glucose control as prescribed by your doctor.
TEPEZZA may cause severe hearing problems including hearing loss, which in some cases may be permanent. Tell your doctor if you have any signs or symptoms of hearing problems or changes in hearing.
Before receiving TEPEZZA, tell your doctor if you:
What are the possible side effects of TEPEZZA?
The most common side effects of TEPEZZA include muscle cramps or spasms, nausea, hair loss, diarrhea, feeling tired, high blood sugar, hearing problems, taste changes, headache, dry skin, weight loss, nail problems, and changes in menstruation.
This is not a complete list of all possible side effects. Tell your doctor or treatment team about any side effect you may have.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/safety/medwatch, or call 1-800-FDA-1088.
Please visit TEPEZZA.com for more information.
Infusion Reactions: TEPEZZA may cause infusion reactions. Infusion reactions have been reported in approximately 4% of patients treated with TEPEZZA. Reported infusion reactions have usually been mild or moderate in severity. Signs and symptoms may include transient increases in blood pressure, feeling hot, tachycardia, dyspnea, headache, and muscular pain. Infusion reactions may occur during an infusion or within 1.5 hours after an infusion. In patients who experience an infusion reaction, consideration should be given to premedicating with an antihistamine, antipyretic, or corticosteroid and/or administering all subsequent infusions at a slower infusion rate.
Preexisting Inflammatory Bowel Disease: TEPEZZA may cause an exacerbation of preexisting inflammatory bowel disease (IBD). Monitor patients with IBD for flare of disease. If IBD exacerbation is suspected, consider discontinuation of TEPEZZA.
Hyperglycemia: Increased blood glucose or hyperglycemia may occur in patients treated with TEPEZZA. In clinical trials, 10% of patients (two-thirds of whom had preexisting diabetes or impaired glucose tolerance) experienced hyperglycemia. Hyperglycemic events should be controlled with medications for glycemic control, if necessary. Assess patients for elevated blood glucose and symptoms of hyperglycemia prior to infusion and continue to monitor while on treatment with TEPEZZA. Ensure patients with hyperglycemia or preexisting diabetes are under appropriate glycemic control before and while receiving TEPEZZA.
Hearing Impairment Including Hearing Loss: TEPEZZA may cause severe hearing impairment including hearing loss, which in some cases may be permanent. Assess patients’ hearing before, during, and after treatment with TEPEZZA and consider the benefit-risk of treatment with patients.
The most common adverse reactions (incidence ≥5% and greater than placebo) are muscle spasm, nausea, alopecia, diarrhea, fatigue, hyperglycemia, hearing impairment, dysgeusia, headache, dry skin, weight decreased, nail disorders, and menstrual disorders.
TEPEZZA is indicated for the treatment of Thyroid Eye Disease regardless of Thyroid Eye Disease activity or duration.
Please see Full Prescribing Information for more information.
WARNINGS AND PRECAUTIONS Infusion Reactions: TEPEZZA may cause infusion reactions. Infusion reactions have been reported in approximately 4% of patients treated with TEPEZZA. Reported infusion reactions have usually been mild or moderate in severity. Signs and symptoms may include transient increases in blood pressure, feeling hot, tachycardia, dyspnea, headache, and muscular pain. Infusion reactions