Get to know Your Thyroid Eye Disease Treatment
Thyroid Eye Disease or TED can be difficult to manage, but you haven't let it stop you. You took control, got answers, and found help.
You and your doctor chose TEPEZZA.
TEPEZZA is a prescription medicine used to treat Thyroid Eye Disease (or TED), no matter if you’ve had TED for months or years.
TEPEZZA may cause infusion reactions. Tell your doctor right away if you have high blood pressure, fast heartbeat, redness of the face or feeling hot, difficulty breathing, headache, or muscle pain.
Please stay tuned for additional Important Safety Information later in this video.
Chapter 1: How TEPEZZA Was Designed to Treat Thyroid Eye Disease
To understand what causes TED, you have to go behind the eye. In the tissue behind the eye are cells that have receptors on their surface that act like switches. When you have TED, your body's immune system attacks this tissue, which causes molecules known as autoantibodies to turn these switches on.
The muscles and the fat tissue swell pushing the eyeball outward, which your doctor may call proptosis. Similarly, it can cause double vision, which your doctor may call diplopia.
TEPEZZA is the first FDA-approved treatment for Thyroid Eye Disease.
TEPEZZA treats a cause of TED, not just the symptoms.
TEPEZZA is designed to bind to the switch and block it from turning on.
Since TEPEZZA blocks the switches so they can't turn on, swelling of the muscle and fat tissue goes down.
This leads to reduced bulging and can also improve double vision and in some cases make it completely go away.
Chapter 2: The Insurance Approval Process
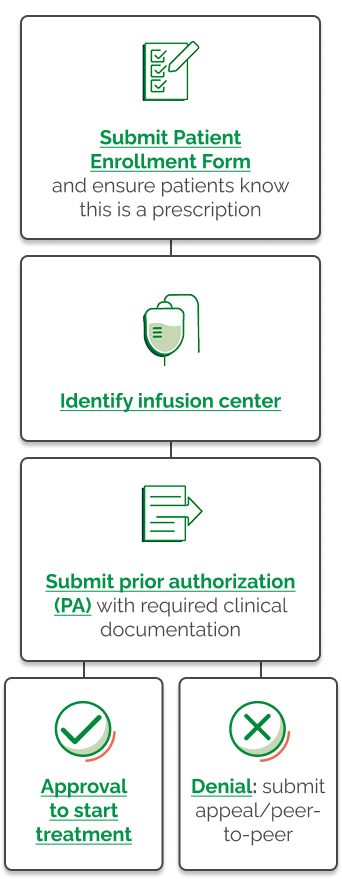
Now that you and your doctor have decided on TEPEZZA, the next step is to gain approval from your insurance provider.
TEPEZZA is a specialty medicine, which means it may require extra insurance approvals.
These approvals require steps like benefits investigation, and prior authorization that usually take between 30 and 90 days, but in some cases, may take longer.
If you're not familiar with these insurance terms, don't worry. In the next chapter of this video, you'll learn about support services available to help you through the TEPEZZA insurance approval process.
The developer of TEPEZZA believes costs should never stop someone from getting the medicine they need.
They're committed to helping you explore options.
That information will be in the next chapter as well.
Chapter 3: Personalized Support with Amgen By Your Side
Amgen By Your Side, a patient support program is here to help.
Once you and your doctor have decided TEPEZZA is right for you, you can sign up to be paired with a Patient Access Liaison or PAL for short.
PALs from the Amgen By Your Side team are available for personal, nonmedical, and logistical assistance throughout your treatment, including discussing what your doctor may have explained about starting treatment, helping to understand the insurance process, explaining financial options, helping you plan your first infusion, and more.
If you're interested, ask your doctor if you can sign the patient enrollment form before it's submitted to Amgen By Your Side. Once enrolled, you will receive a call from your PAL.
Chapter 4: Getting your TEPEZZA Infusion
TEPEZZA is an IV or intravenous medicine. This means it's delivered through a needle placed in your arm. The process of delivering an IV medicine into the body is called an infusion.
A full course of TEPEZZA treatment is 8 infusions. Each infusion is given one time every three weeks. That means the treatment with TEPEZZA will be ongoing for about five months.
TEPEZZA may be given at an infusion center, a type of clinic that specializes in giving infusions.
It may also be given at a doctor's office, hospital or at home.
Check with both your insurance provider and your doctor to see if home infusion may be an option for you.
For your first infusion, having someone take you to and from the appointment is recommended.
When it's time to start your TEPEZZA treatment, you'll be taken to an infusion chair, which is a lot like a recliner. Once the IV is in, all you have to do is relax and receive the infusion.
Each infusion appointment usually lasts one and a half to two and a half hours, but will vary depending on where you get your infusion. During this time, books, music and movies can all provide a welcome distraction.
I was very scared.
They would make me very comfortable. I didn't even think of it as oh my gosh, I'm sitting here getting an infusion. This is terrible. I thought, hey, you know, this isn't bad.
Chapter 5: The Possible Side Effects of TEPEZZA
Here's a list of the most common side effects of TEPEZZA and the percentage of people that experienced them in clinical studies.
- Muscle cramps or spasms, 25% of patients;
- nausea, 17%;
- hair loss, 13%;
- diarrhea, 12%;
- tiredness, 12%;
- high blood sugar, 10%;
- hearing problems, 10%;
- changes in taste, 8%;
- headache, 8%;
- dry skin, 8%;
- weight loss, 6%;
- nail problems, 5%;
- changes in menstruation, 23%.
Always reach out to your doctor to discuss how to manage side effects.
Chapter 6: How TEPEZZA Can Help Thyroid Eye Disease
Some people had less eye bulging as soon as six weeks after starting treatment with TEPEZZA, and improvement continued over the full treatment course of 8 infusions.
Once you start seeing results with TEPEZZA, you may want to stop receiving infusions, but it is recommended you receive all 8 of them.
It was a slow process, but the bulging became less. The double vision was gone. To not have the bulgy eyes, it's really nice.
Follow TEPEZZA on Facebook or Instagram to join the community, get important updates, and stay connected.
Congratulations on taking the first step with TEPEZZA.
Talk to your doctor about TEPEZZA.