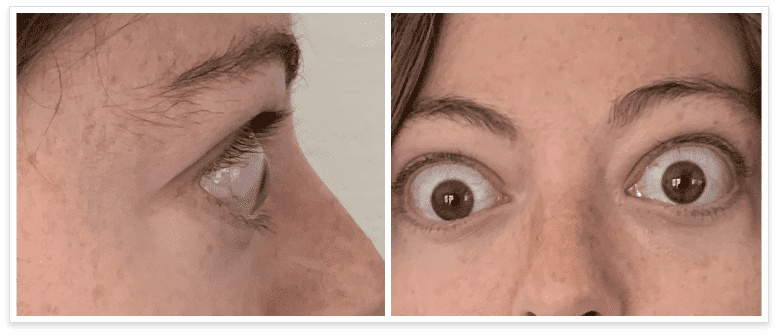
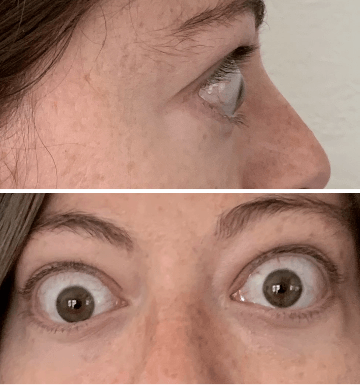
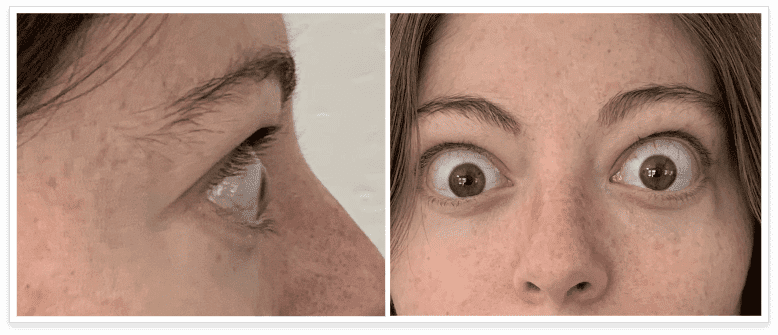
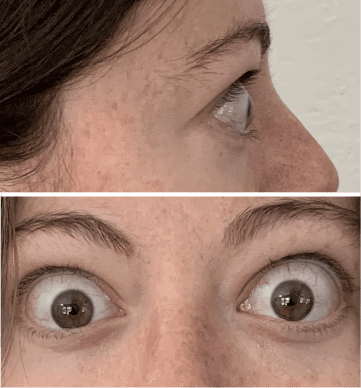
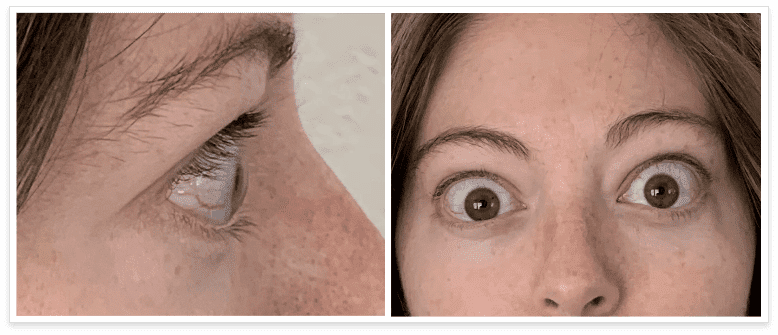
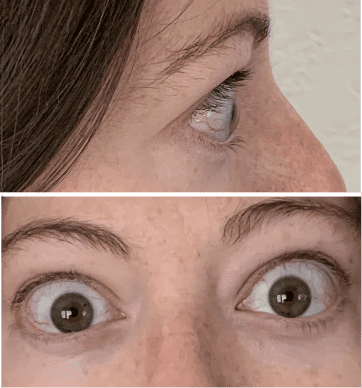
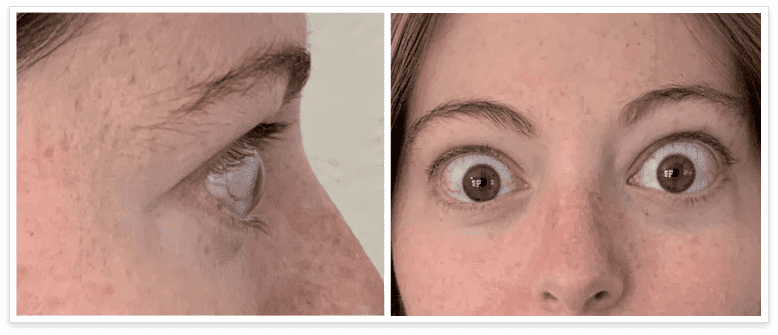
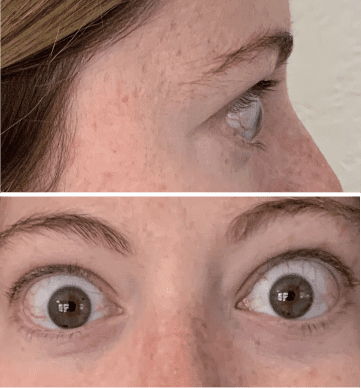
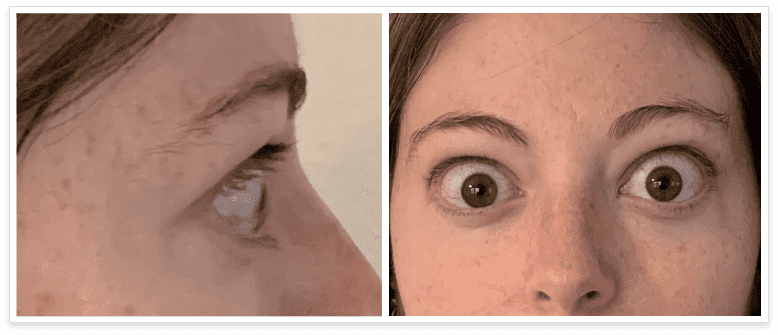
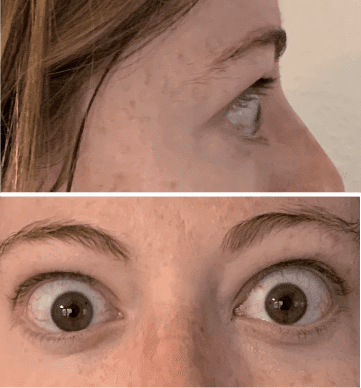
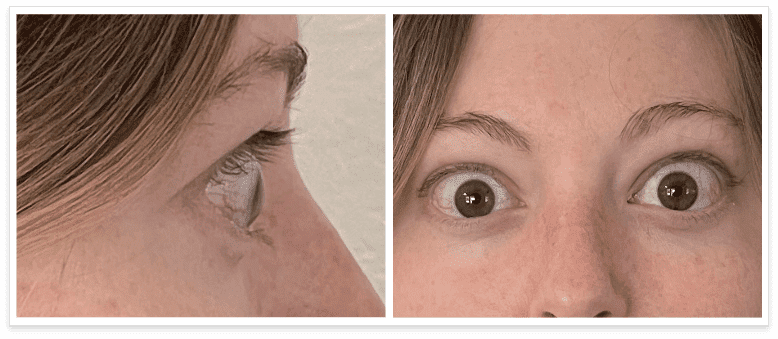
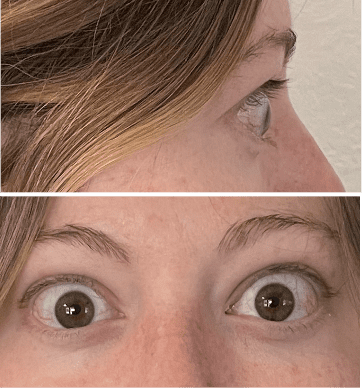
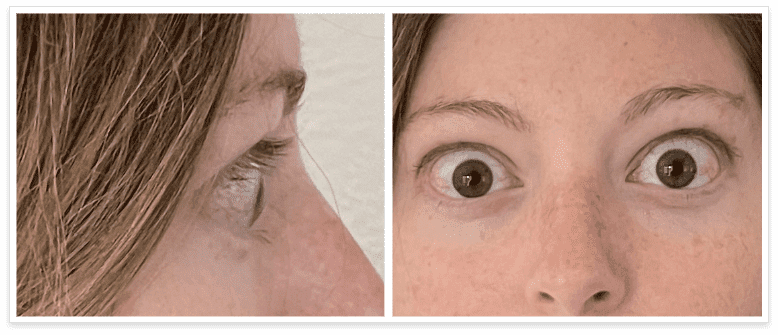
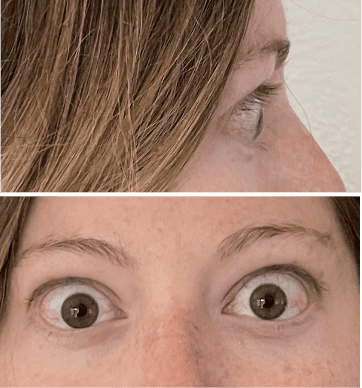
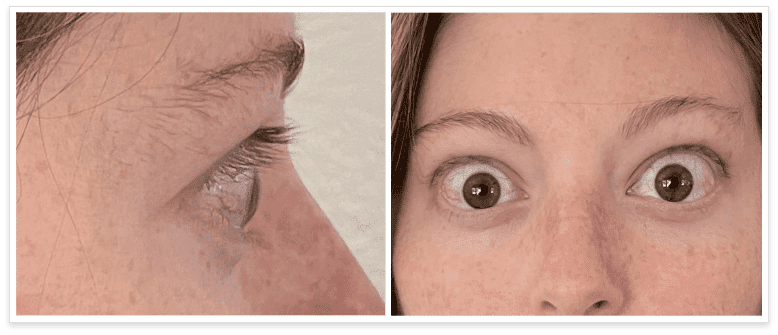
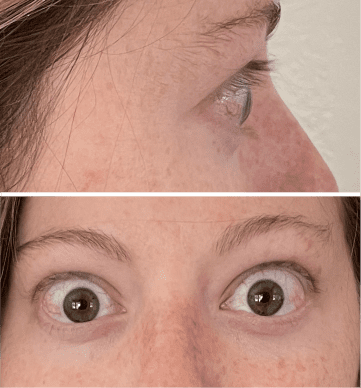
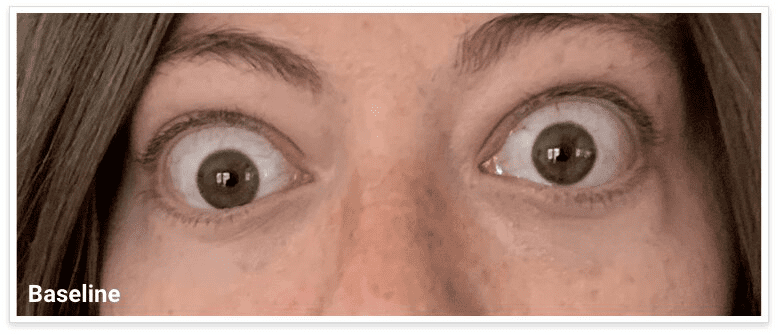
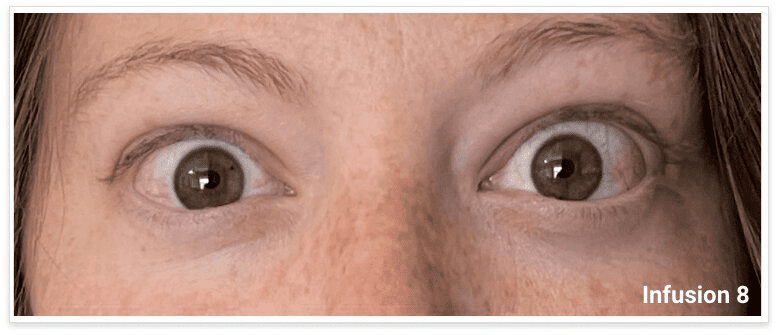
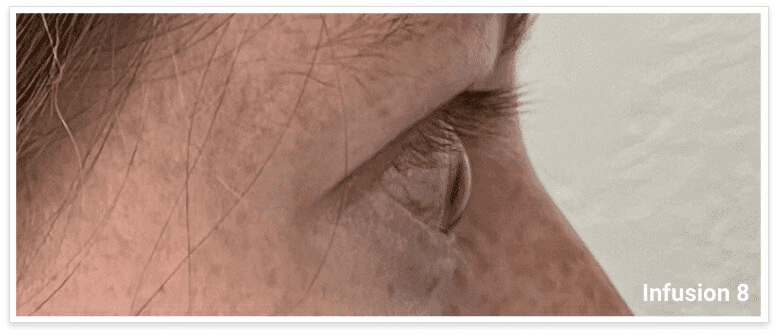
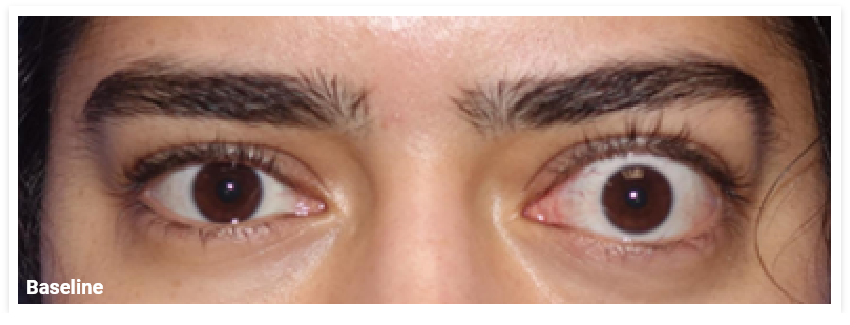
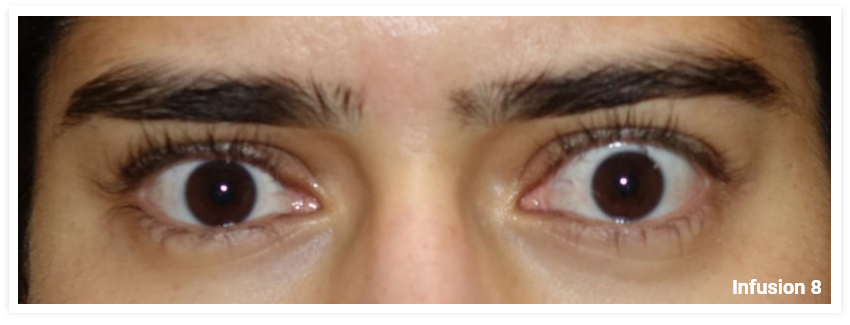
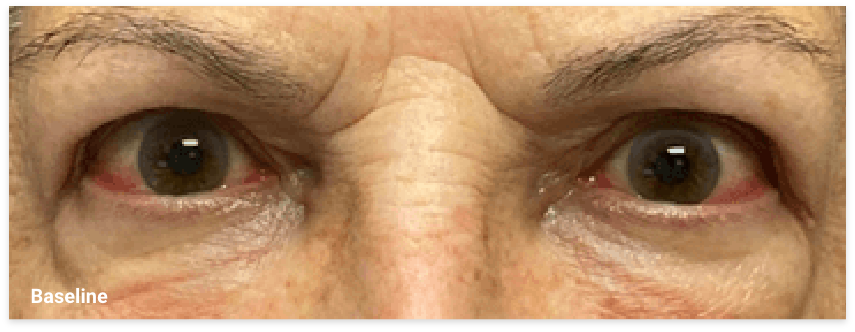
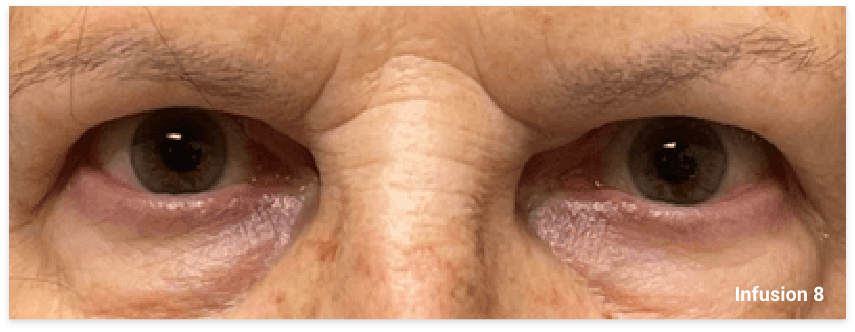
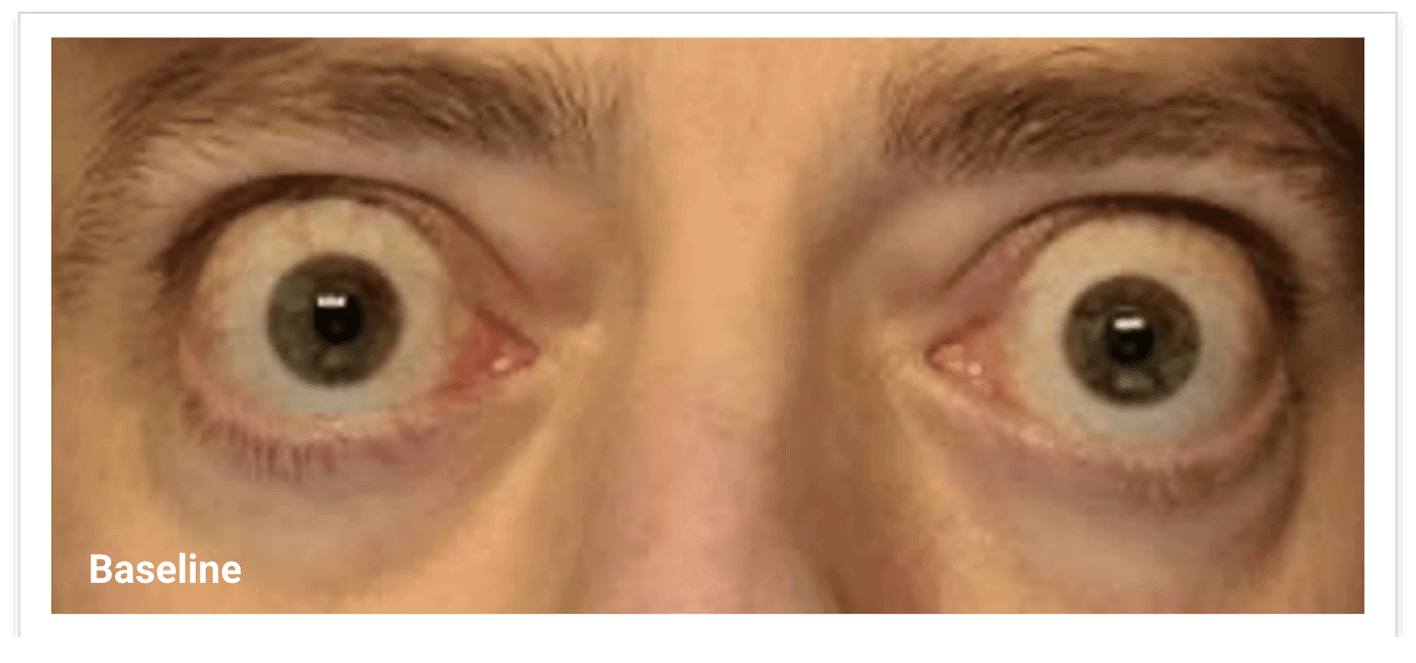
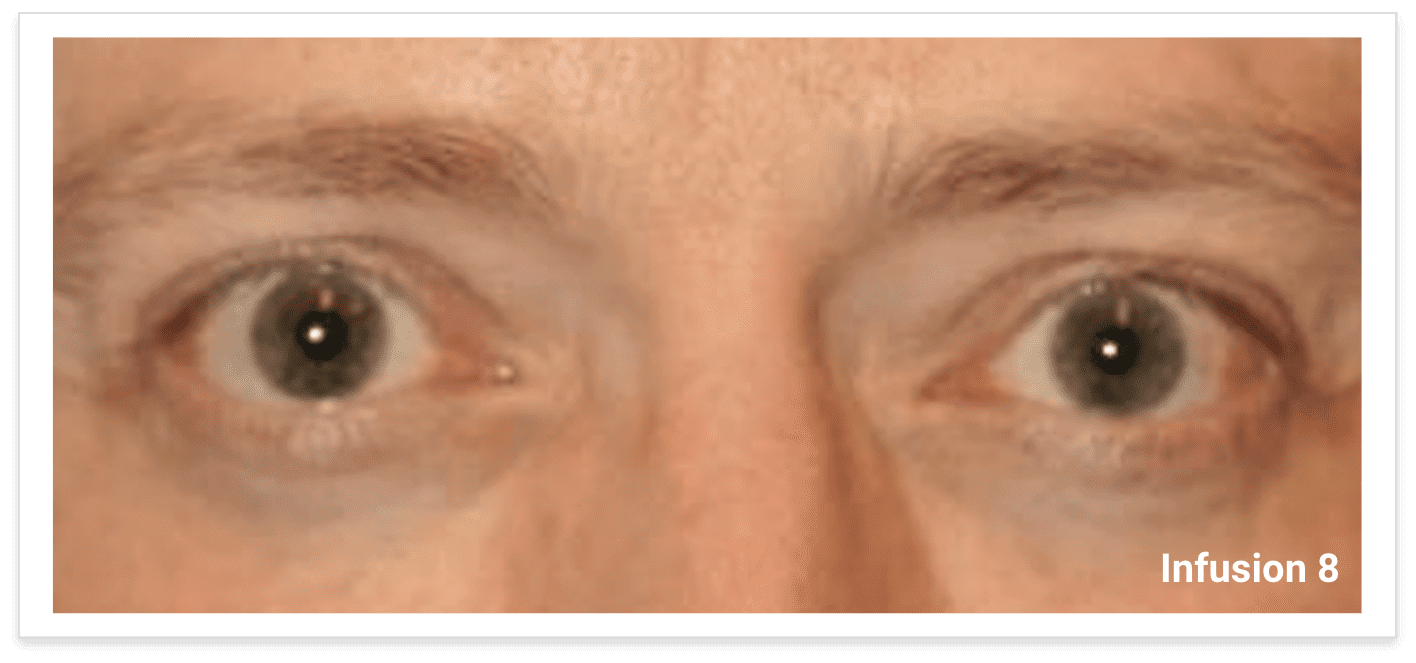
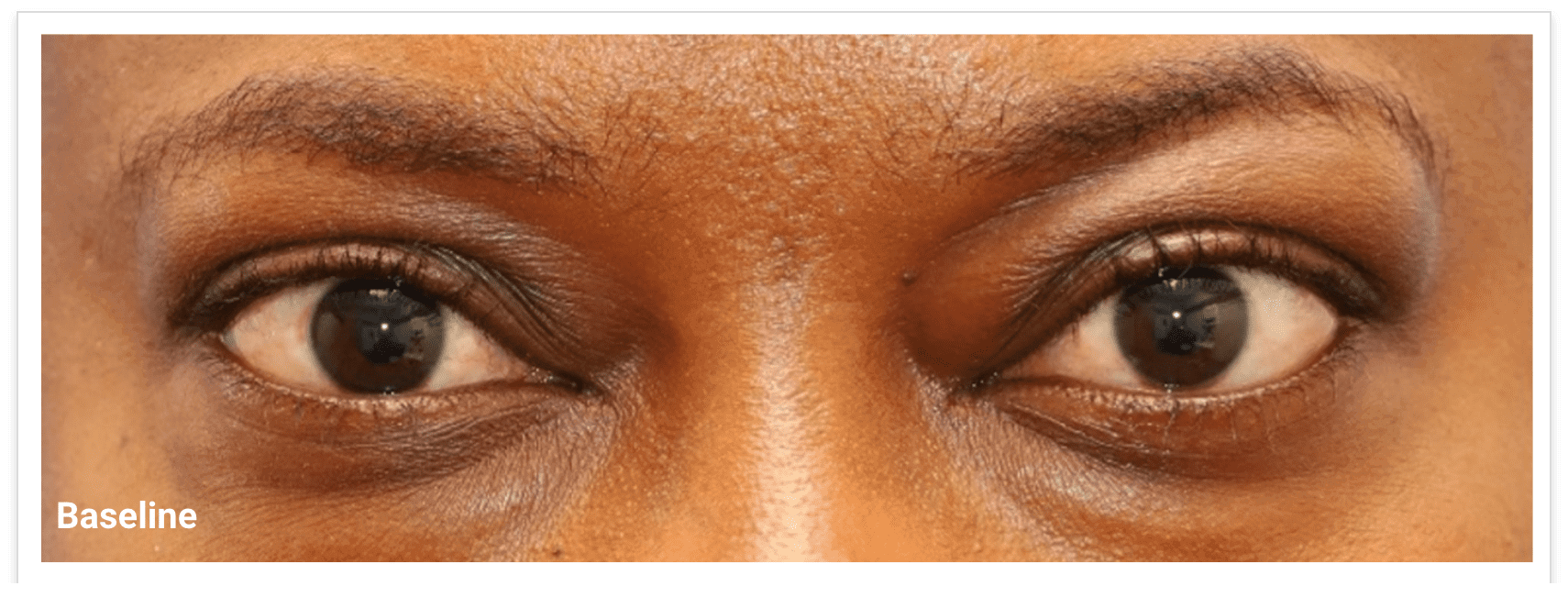
My name is Bonnie, and I was diagnosed with Thyroid Eye Disease in August of 2020. After trying steroids and not having any results in the reduction of my symptoms, my doctor and I decided that TEPEZZA was the best option for me.
INDICATION
TEPEZZA is indicated for the treatment of Thyroid Eye Disease regardless of Thyroid Eye Disease activity or duration.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Infusion Reactions: TEPEZZA may cause infusion reactions. Infusion reactions have been reported in approximately 4% of patients treated with TEPEZZA. Reported infusion reactions have usually been mild or moderate in severity. Signs and symptoms may include transient increases in blood pressure, feeling hot, tachycardia, dyspnea, headache, and muscular pain. Infusion reactions may occur during an infusion or within 1.5 hours after an infusion. In patients who experience an infusion reaction, consideration should be given to premedicating with an antihistamine, antipyretic, or corticosteroid and/or administering all subsequent infusions at a slower infusion rate.
Preexisting Inflammatory Bowel Disease: TEPEZZA may cause an exacerbation of preexisting inflammatory bowel disease (IBD). Monitor patients with IBD for flare of disease. If IBD exacerbation is suspected, consider discontinuation of TEPEZZA.
Hyperglycemia: Increased blood glucose or hyperglycemia may occur in patients treated with TEPEZZA. In clinical trials, 10% of patients (two-thirds of whom had preexisting diabetes or impaired glucose tolerance) experienced hyperglycemia. Hyperglycemic events should be controlled with medications for glycemic control, if necessary. Assess patients for elevated blood glucose and symptoms of hyperglycemia prior to infusion and continue to monitor while on treatment with TEPEZZA. Ensure patients with hyperglycemia or preexisting diabetes are under appropriate glycemic control before and while receiving TEPEZZA.
Hearing Impairment Including Hearing Loss: TEPEZZA may cause severe hearing impairment including hearing loss, which in some cases may be permanent. Assess patients’ hearing before, during, and after treatment with TEPEZZA and consider the benefit-risk of treatment with patients.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥5% and greater than placebo) are muscle spasm, nausea, alopecia, diarrhea, fatigue, hyperglycemia, hearing impairment, dysgeusia, headache, dry skin, weight decreased, nail disorders, and menstrual disorders.