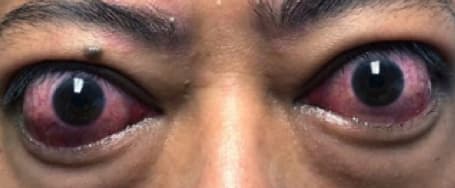
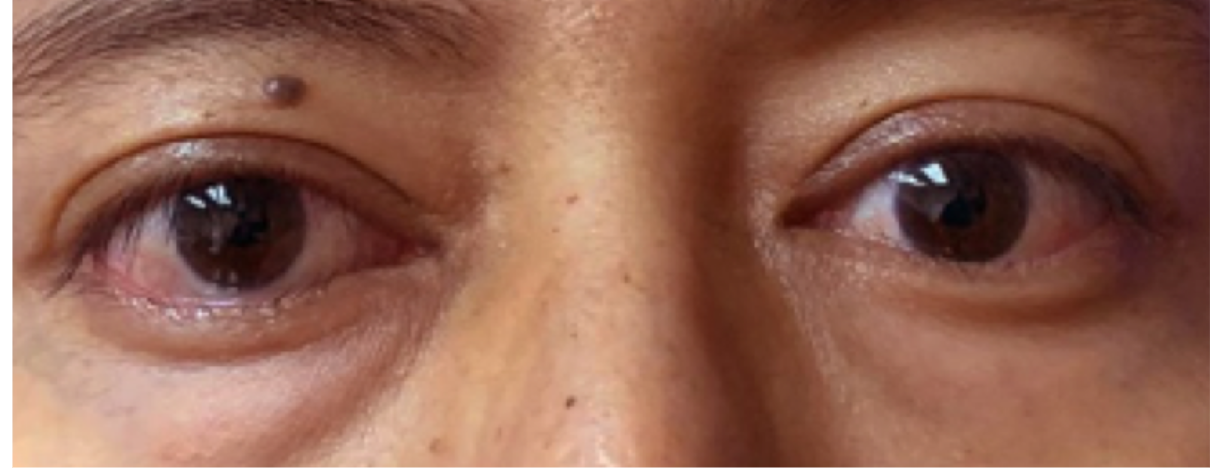
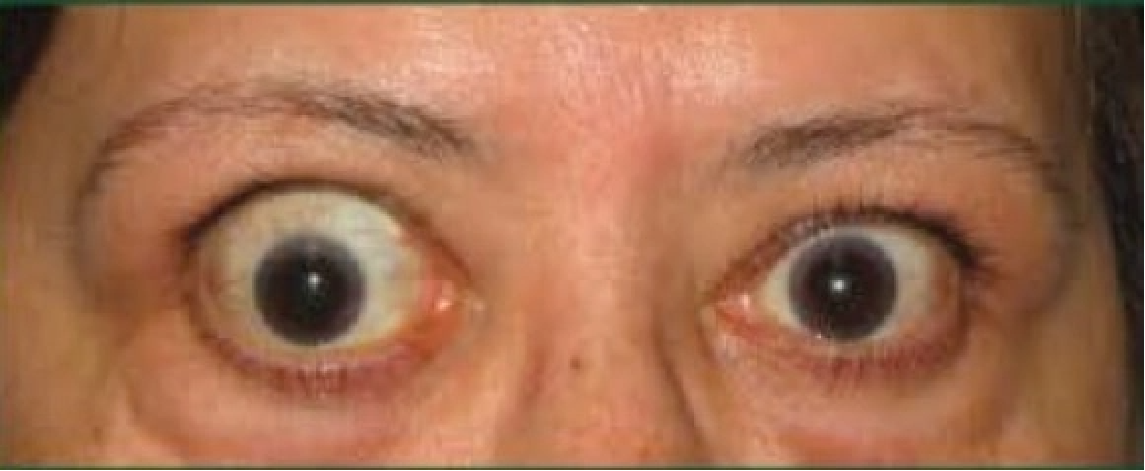
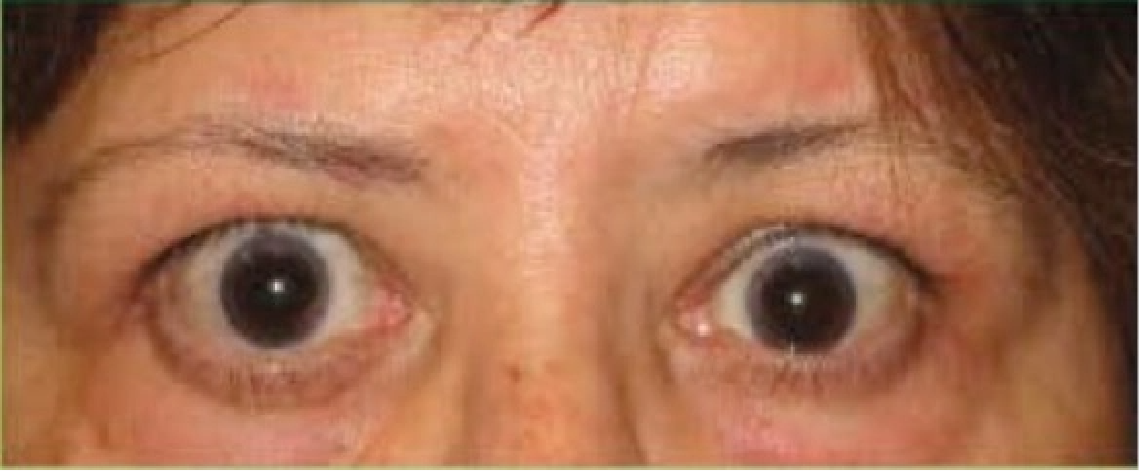
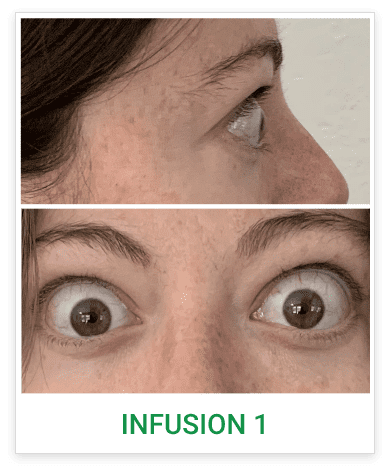
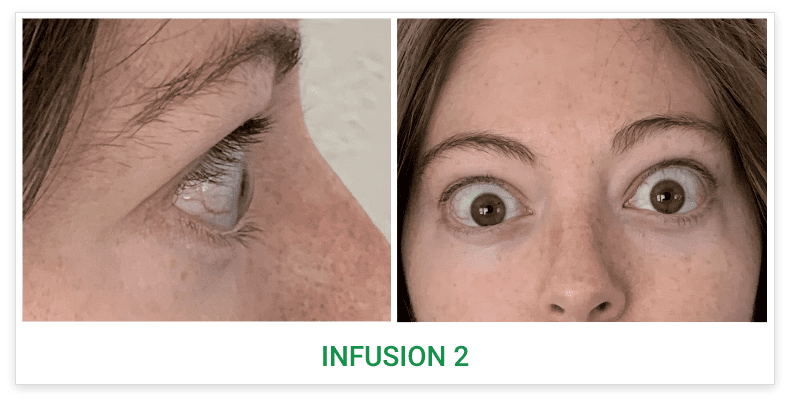
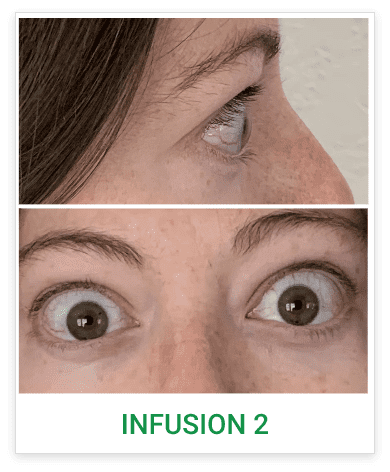
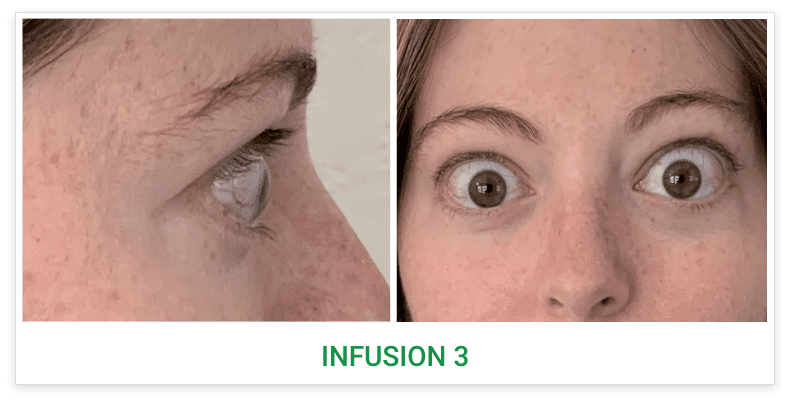
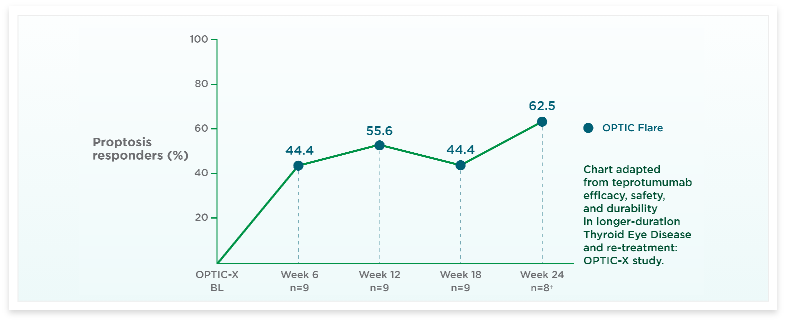
See results for patients treated with TEPEZZA in Phase 2/3 clinical trials
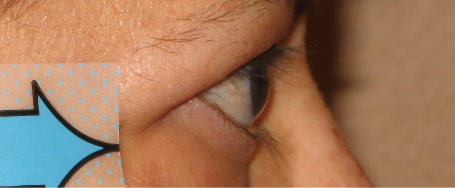
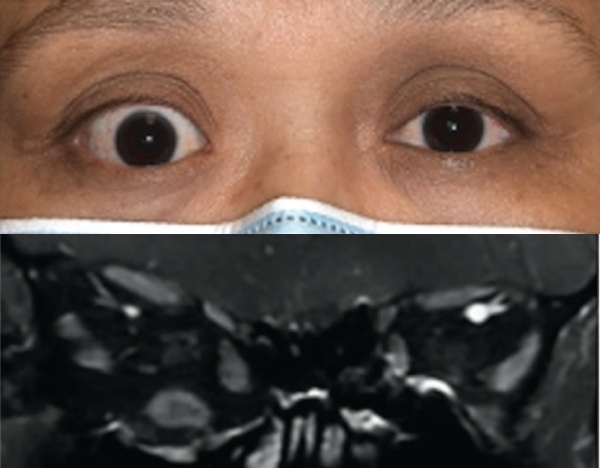
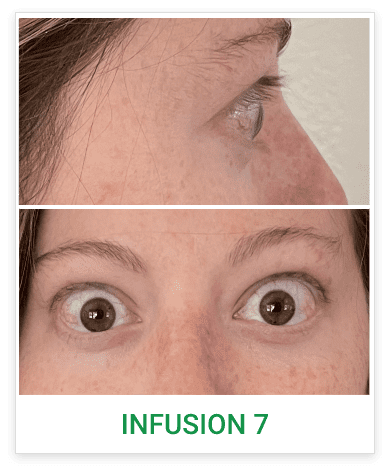
Baseline3
Proptosis: 24 mm
Diplopia: 0
Clinical Activity Score (CAS): 5
Inflammatory signs and symptoms:
- Eyelid swelling
- Eyelid erythema
- Conjunctival redness
- Chemosis
- Inflammation of caruncle/plica
Post-treatment (Week 24)3
Proptosis: 19 mm
Diplopia: 0
CAS: 1
Inflammatory signs and symptoms:
- Eyelid swelling
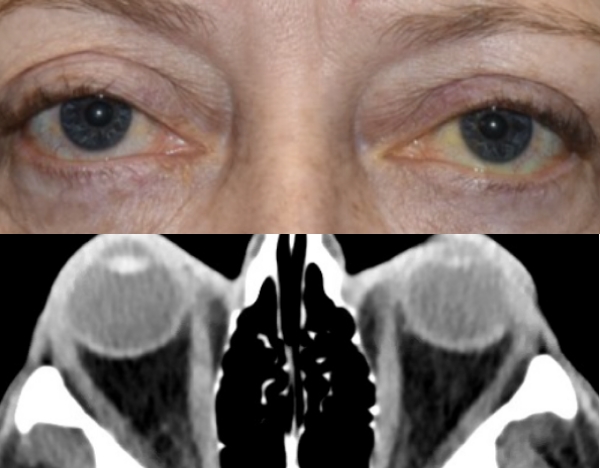
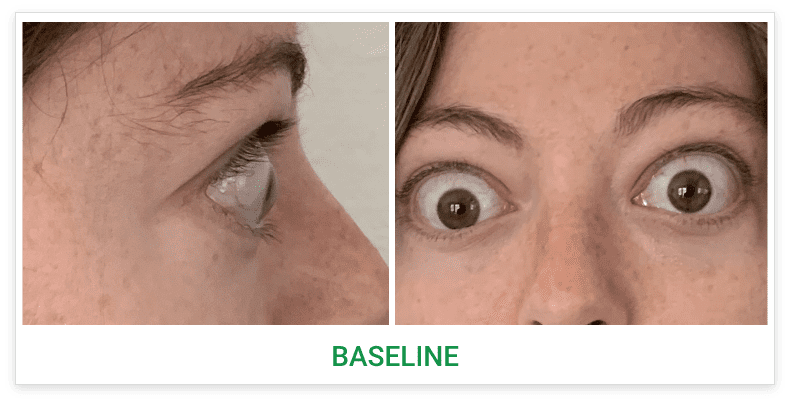
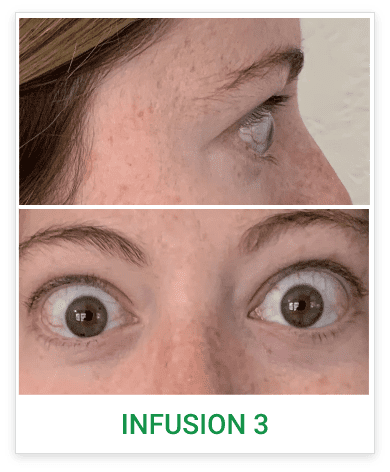
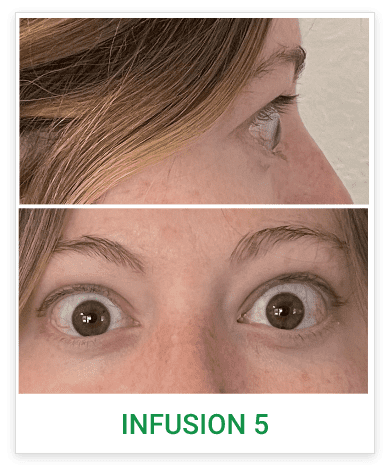
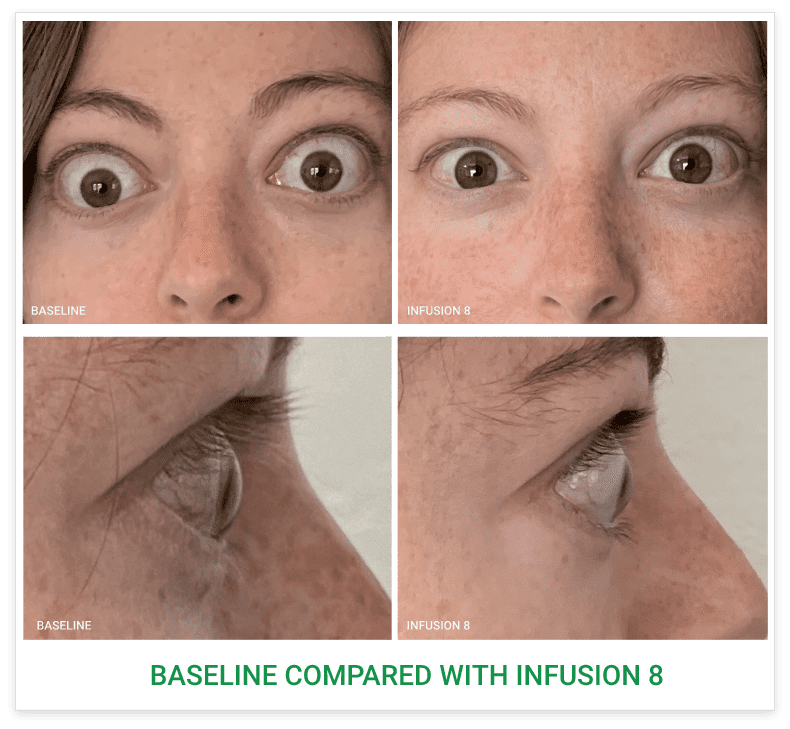
Baseline5
Proptosis: 25 mm
Diplopia: 3
CAS: 5
Inflammatory signs and symptoms:
- Spontaneous orbital pain
- Gaze-evoked orbital pain
- Eyelid swelling
- Eyelid erythema
- Conjunctival redness
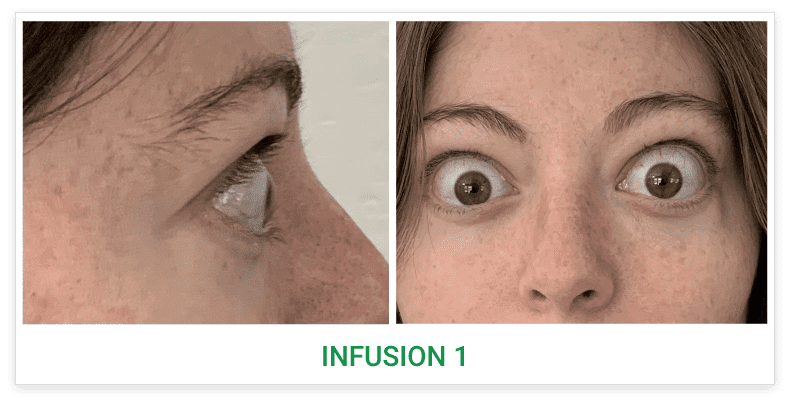
Post-treatment (Week 24)5
Proptosis: 21 mm
Diplopia: 0
CAS: 0
Inflammatory signs and symptoms:
- No inflammatory signs or symptoms
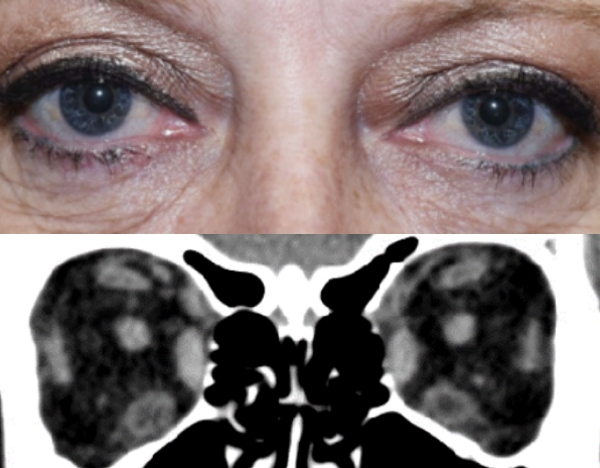
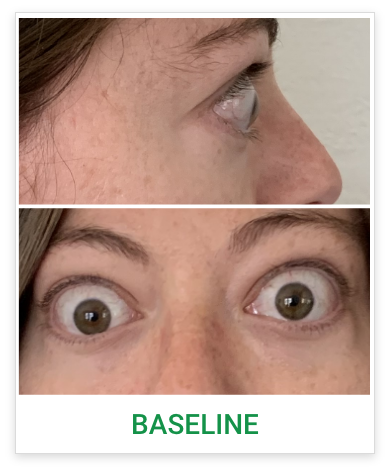
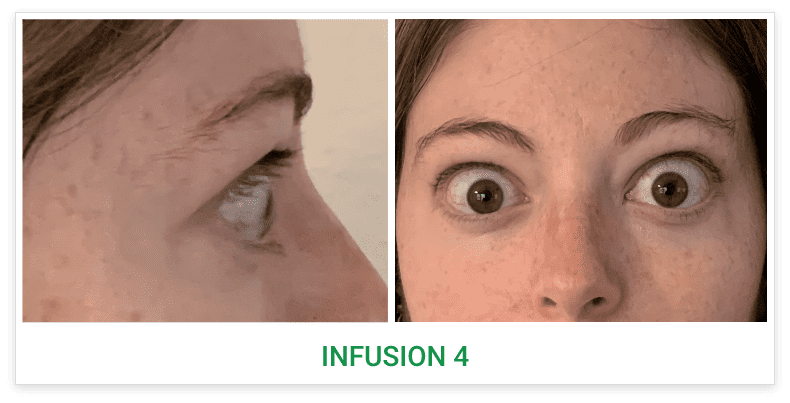
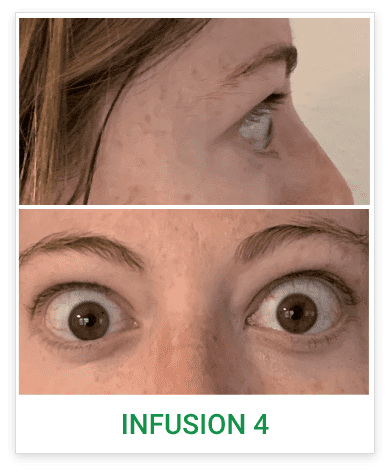
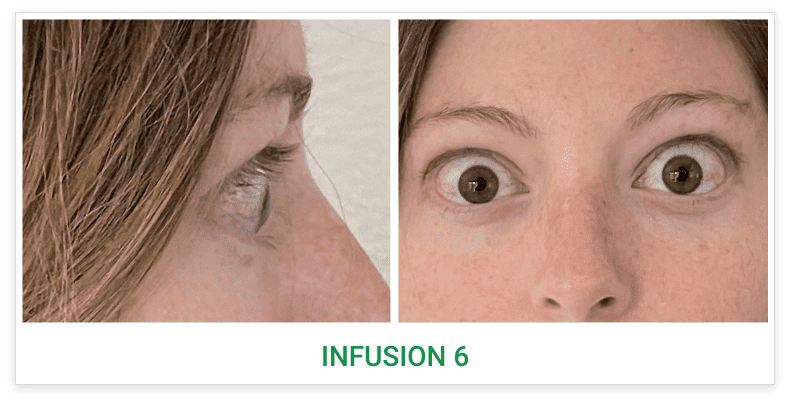
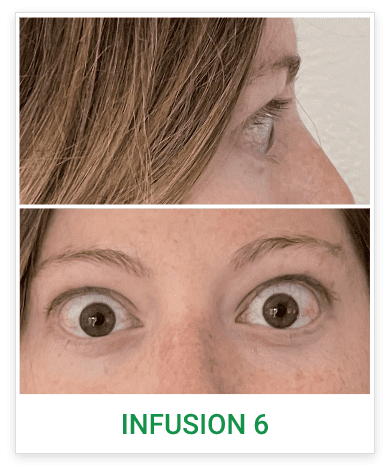
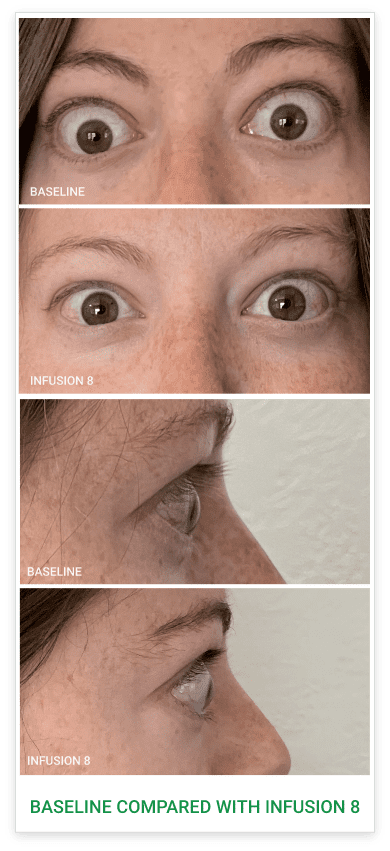
Baseline5
Proptosis: 25 mm
Diplopia: 3
CAS: 5
Inflammatory signs and symptoms:
- Spontaneous orbital pain
- Gaze-evoked orbital pain
- Eyelid swelling
- Eyelid erythema
- Conjunctival redness
Post-treatment (Week 24)5
Proptosis: 21 mm
Diplopia: 0
CAS: 0
Inflammatory signs and symptoms:
- No inflammatory signs or symptoms
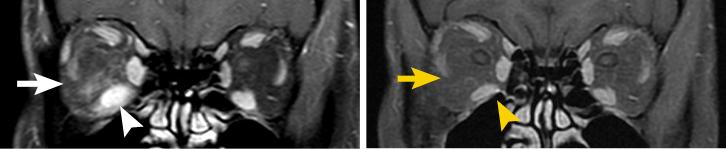
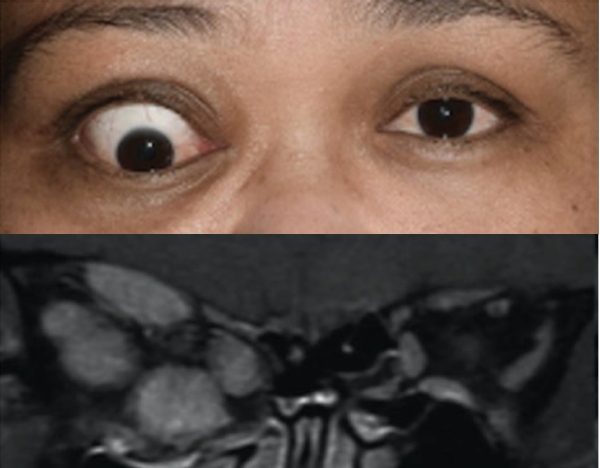
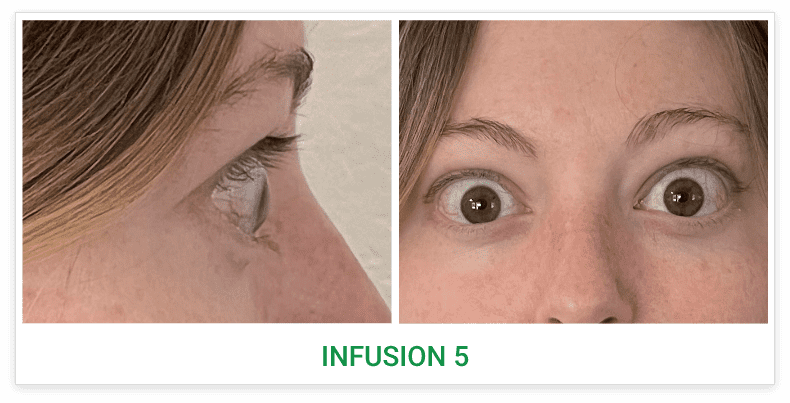
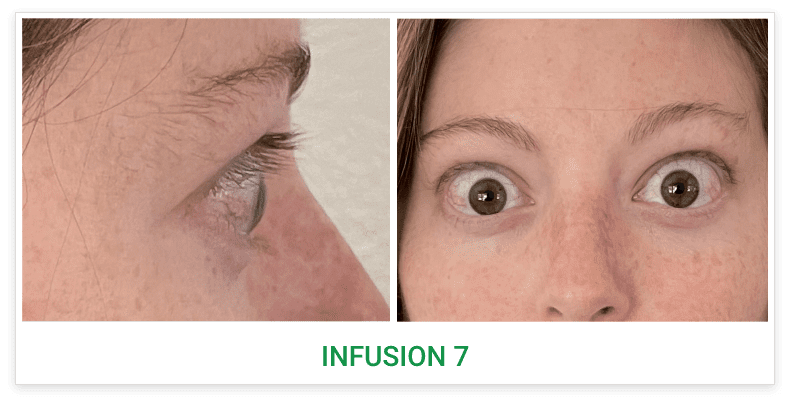
Baseline5
Proptosis: 23 mm
Diplopia: 3
Inflammatory signs and symptoms:
- Gaze-evoked orbital pain
- Eyelid swelling
- Eyelid erythema
- Conjunctival redness
- Chemosis
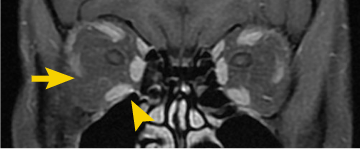
Post-treatment (Week 24)5
Proptosis: 18 mm
Diplopia: 0
Inflammatory signs and symptoms:
- No inflammatory signs or symptoms
Patient treated with TEPEZZA in a clinical trial.5 At baseline, inferior rectus muscle (white arrowhead) and orbital fat (white arrow) are enhanced. Muscle and fat reduction at Week 24 includes: reduction of orbital fat (yellow arrow), reduction of the size of the inferior rectus muscle by 49% according to volumetric analysis (yellow arrowhead), and reduction in volume of the medial rectus muscle by 41%.2
Shown above are coronal, contrast-enhanced, fat-saturated, T1-weighted MRI scans from a patient in the teprotumumab group.2 Adapted from Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. Copyright Massachusetts Medical Society.